Recommendation for individuals using a screenreader: please set your punctuation settings to "most."
Recommendation for individuals using a screenreader: please set your punctuation settings to "most."
Descriptive Statements:
- Analyze the three laws of thermodynamics and their applications to chemical and biochemical systems.
- Predict the spontaneity of given chemical reactions.
- Differentiate among forms of energy and between heat and temperature.
- Analyze the results of calorimetry experiments.
Sample Item:
A 4.75 g sample of solid NaOH is dissolved in 50.5 g of H2O in a constant-pressure
calorimeter having a heat capacity of 18.5 J/°C18.5 joules per degrees Celsius. The temperature rises from 21.1°C21.1 degrees Celsius
to 33.6°C33.6 degrees Celsius. Assuming that the solution has a specific heat capacity of 4.184 J/g·°C 4.184 joules per gram per degrees Celsius
and negligible heat loss from the calorimeter, how much heat is produced in the solution
process?
- 2.64 kJ
- 2.89 kJ
- 3.12 kJ
- 4.27 kJ
Correct Response and Explanation (Show Correct ResponseHide Correct Response)
C. This question requires the examinee to analyze the results of a
calorimetry experiment. The total heat produced in the given solution process is equal
to the heat absorbed by the solution plus the heat absorbed by the calorimeter. The heat
absorbed by the solution is calculated using the equation q = m × s × ΔTq equals m times s times Delta T. The heat
absorbed by the calorimeter is equal to Ccalorimeter × ΔTC subscript calorimeter times Delta T.
Descriptive Statements:
- Analyze energy changes due to the formation or breaking of chemical bonds.
- Analyze energy changes during chemical reactions, including the analysis of enthalpy diagrams.
- Analyze energy changes involved in phase transitions, dissolving solutes in solvents, and diluting solutions.
Sample Item:
| Bond |
Bond Enthalpy
(kJ/mol) |
| H—H |
436.4 |
| H—Cl |
431.9 |
H2(g) + Cl2(g) → 2HCl(g)
ΔH °rxn = –184.6 kJH2(g) plus Cl2(g) right arrow 2HCl(g) new line
Delta H degrees subscript rxn equals negative 184.6 kJ
Given the information shown above, what is the best estimate of the bond enthalpy for
the Cl—Cl bond?
- 189.1 kJ/mol
- 242.8 kJ/mol
- 256.3 kJ/mol
- 585.1 kJ/mol
Correct Response and Explanation (Show Correct ResponseHide Correct Response)
B. This question requires the examinee to analyze energy changes due to
the formation or breaking of chemical bonds. The enthalpy change for a chemical reaction
is equal to the sum of the enthalpy changes involved in breaking existing bonds minus the
sum of the enthalpy changes involved in forming new bonds. The bond enthalpy for the
Cl—Cl bond can be calculated using this relationship and the given bond enthalpies for
H—H and H—Cl.
Descriptive Statements:
- Apply the International Union of Pure and Applied Chemistry (IUPAC) rules of nomenclature.
- Analyze the characteristics of inorganic structures, including ionic solids, network solids, and metallic solids.
- Predict the geometry of molecules and polyatomic ions.
- Analyze the chemical composition and basic structure of organic compounds.
- Recognize the characteristics of structural, geometric, and optical isomers.
Sample Item:
Which of the following structural formulas represents 4-ethyl-3, 3-dimethylhexane?
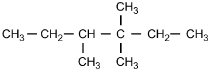
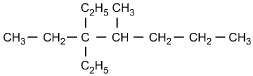
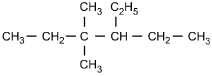
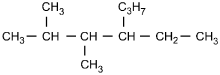
Correct Response and Explanation (Show Correct ResponseHide Correct Response)
C. This question requires the examinee to apply the International Union
of Pure and Applied Chemistry (IUPAC) rules of nomenclature. 4-ethyl-3, 3-dimethylhexane
is an alkane consisting of six continuous carbon atoms. An ethyl group (C2H5)
is attached to the number 4 carbon and two methyl groups (CH3) are attached to the
number 3 carbon.
Descriptive Statements:
- Compare the characteristics of types of chemical bonds.
- Analyze chemical bonding in terms of electron behavior and the factors that affect bond strength.
- Analyze the characteristics of various types of intermolecular forces and the forces between molecules of a given structure.
- Relate the properties of substances to their atomic bonds and intermolecular forces.
Sample Item:
The high melting point of diamond is due to:
- strong covalent bonds between carbon atoms.
- an irregular, three-dimensional crystal structure.
- delocalized, highly mobile bonding electrons.
- extensive van der Waals forces between carbon atoms.
Correct Response and Explanation (Show Correct ResponseHide Correct Response)
A. This question requires the examinee to relate the properties of
substances to their atomic bonds. Diamond is a covalent-network crystalline solid.
The carbon atoms in this network are linked by covalent bonds. This strong bonding
between carbon atoms is responsible for the high melting point of diamond.